Chemistry, 03.02.2020 22:51 corrineikerd
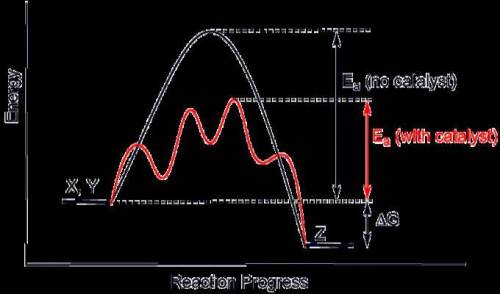
Acatalyst lowers the activation energy for both the forward and the reverse reactions in an equilibrium system, so it has no effect on the equilibrium position of a system. true false

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:00
Write a hypothesis that answers the lesson question, “while observing a chemical reaction, how can you tell which reactant is limiting? ” hypothesis: if a substance is the limiting reactant, then . . because . .
Answers: 1

Chemistry, 22.06.2019 10:30
Which describes fat? a: a carbohydrate that produces energy b: a nucleic acid that directs cell function c: a lipid that stores energy d: a protein that speeds up a chemical reaction
Answers: 1

Chemistry, 22.06.2019 12:10
Building glycogen from glucose molecules is an example of
Answers: 3

Chemistry, 23.06.2019 01:00
Reactions in cells take place at about a. 40°c b. 0° c. 100°c d. 60°c
Answers: 1
You know the right answer?
Acatalyst lowers the activation energy for both the forward and the reverse reactions in an equilibr...
Questions



Mathematics, 30.11.2020 21:40


Advanced Placement (AP), 30.11.2020 21:40



Physics, 30.11.2020 21:40



Mathematics, 30.11.2020 21:40





History, 30.11.2020 21:40

Health, 30.11.2020 21:40

English, 30.11.2020 21:40