
Chemistry, 06.10.2019 07:11 kberly3750ovgw6f
According to the following balanced equation, 2 formula units of iron (iii) oxide (fe2o3) can be formed by reacting 4 atoms of iron (fe) with 3 molecules of oxygen gas (o2). if 12 atoms of iron are reacted with 6 molecules of oxygen gas, which is the limiting reactant and how many atoms or molecules will be left over? 4fe + 3o2 -> 2fe2o3

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Esign techniques and materials that reduce the negative environmental impact of a structure are referred to as
Answers: 3

Chemistry, 22.06.2019 13:00
Which of the following are good traits of a hypothesis? it will be able to be testedit can predict an outcomeit will explain the observationsall of these
Answers: 2

Chemistry, 22.06.2019 16:00
How does blood clotting prevent the entry of pathogens through cuts and wounds? answer asap,, this is due tomorrow. will mark as brainliest or whatever you call it : )
Answers: 2

Chemistry, 22.06.2019 21:30
Which of the following changes will decrease the total amount of gaseous solute able to be dissolved in a liter of liquid water? (2 points) decreasing temperature decreasing pressure decreasing surface area decreasing solute concentration
Answers: 1
You know the right answer?
According to the following balanced equation, 2 formula units of iron (iii) oxide (fe2o3) can be for...
Questions

Mathematics, 18.05.2021 18:40




Mathematics, 18.05.2021 18:40


Mathematics, 18.05.2021 18:40

Chemistry, 18.05.2021 18:40


Chemistry, 18.05.2021 18:40

Social Studies, 18.05.2021 18:40


Mathematics, 18.05.2021 18:40

Biology, 18.05.2021 18:40





Mathematics, 18.05.2021 18:40

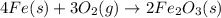
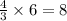 atoms of iron.
atoms of iron.

