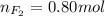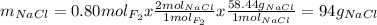Part 1. a chemist reacted 18.0 liters of f2 gas with nacl in the laboratory to form cl2 gas and naf. use the ideal gas law equation to determine the mass of nacl that reacted with f2 at 290. k and 1.5 atm.
f2 + 2nacl → cl2 + 2naf
part 2. explain how you would determine the mass of sodium chloride that can react with the same volume of fluorine gas at stp.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
After cloud droplets form, what must happen to them for precipitation to occur?
Answers: 1

Chemistry, 22.06.2019 08:00
Me i dont know what to do! the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1

Chemistry, 22.06.2019 14:30
How does a noncompetitive inhibitor reduce an enzyme’s activity?
Answers: 1

Chemistry, 22.06.2019 16:30
For the reaction shown, calculate how many moles of no2 form when each of the following completely reacts. 2n2o5(g)→4no2(g)+o2(g) part a 1.0 mol n2o5 express your answer using two significant figures. nothing mol m o l request answer part b 5.4 mol n2o5 express your answer using two significant figures.
Answers: 2
You know the right answer?
Part 1. a chemist reacted 18.0 liters of f2 gas with nacl in the laboratory to form cl2 gas and naf....
Questions


Mathematics, 26.10.2020 04:50


History, 26.10.2020 04:50

Mathematics, 26.10.2020 04:50


Mathematics, 26.10.2020 04:50



Arts, 26.10.2020 04:50



English, 26.10.2020 04:50

Mathematics, 26.10.2020 04:50

Biology, 26.10.2020 04:50



Mathematics, 26.10.2020 04:50


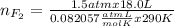 →
→ 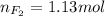
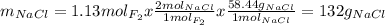
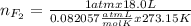 →
→