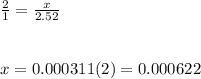Chemistry, 04.02.2020 14:57 alexis3744
Achemistry student needs to standardize a fresh solution of sodium hydroxide. he carefully weighs out 28.mg of oxalic acid h2c2o4 , a diprotic acid that can be purchased inexpensively in high purity, and dissolves it in 250.ml of distilled water. the student then titrates the oxalic acid solution with his sodium hydroxide solution. when the titration reaches the equivalence point, the student finds he has used 28.4ml of sodium hydroxide solution. calculate the molarity of the student's sodium hydroxide solution. be sure your answer has the correct number of significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:20
If i can still dissolve more sugar into the solution at a certain temperature what would i call that solution
Answers: 3

Chemistry, 22.06.2019 10:30
Geothermal energy for industrial use is available almost anywhere. a.true b.false
Answers: 2

Chemistry, 22.06.2019 10:40
Asolid that forms and separates from a liquid mixture is called
Answers: 2

You know the right answer?
Achemistry student needs to standardize a fresh solution of sodium hydroxide. he carefully weighs ou...
Questions

Chemistry, 20.08.2019 09:30

History, 20.08.2019 09:30


Physics, 20.08.2019 09:30



Health, 20.08.2019 09:30


English, 20.08.2019 09:30

History, 20.08.2019 09:30

Chemistry, 20.08.2019 09:30


English, 20.08.2019 09:30

History, 20.08.2019 09:30



Mathematics, 20.08.2019 09:30

Health, 20.08.2019 09:30


Mathematics, 20.08.2019 09:30