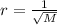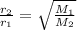Chemistry, 05.02.2020 11:45 bigbrogamer
(~40 points, answer and explain)
-what is the order of increasing rate of effusion for the following gases? ar, co2, he, n2 (in the form of a< b< c< d)
-place the following gases in order of increasing speed at the same temperature. n2, h2, cl2, co2, ar

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 22.06.2019 15:00
Describe what happens to the molecules as water goes from ice to liquid to vapor. be sure to explain what happens to the temperature during the phase changes.
Answers: 2

Chemistry, 22.06.2019 23:00
Consider the reaction: 2al(s) + fe2o3(s) → al2o3(s) + 2fe(s) the δhf for fe2o3(s) = -824.3 kj/mole. the δhf for al2o3(s) = -1675.7 kj/mole. finish the equation. δhrxn = [(1)( kj/mole) + (2)( kj/mole)] - [(1)( kj/mole) + (2) ( kj/mole)]
Answers: 1
You know the right answer?
(~40 points, answer and explain)
-what is the order of increasing rate of effusion for t...
-what is the order of increasing rate of effusion for t...
Questions


Mathematics, 02.03.2021 05:00

History, 02.03.2021 05:00

Physics, 02.03.2021 05:00






Mathematics, 02.03.2021 05:00


Mathematics, 02.03.2021 05:00



History, 02.03.2021 05:00

Biology, 02.03.2021 05:00

Mathematics, 02.03.2021 05:00



Mathematics, 02.03.2021 05:00