
Chemistry, 30.01.2020 17:03 Isaiahtate053
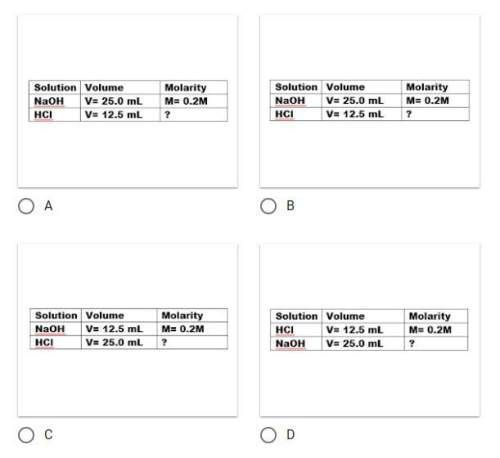
You have 25.0ml hcl of unknown concentration. it takes 12.5 ml of 0.2 m naoh to neutralize the acid. determine the concentration of hcl in the following steps.
which data table would you use to organize the information correctly?

Answers: 1


Another question on Chemistry


Chemistry, 21.06.2019 22:50
Blank allows you to do calculations for situations in which only the amount of gas is constant a)boyle's law b)combined gas law c)ideal gas law d)dalton's law
Answers: 1


Chemistry, 22.06.2019 21:00
What is the chemical formula for the compound formed between sodium and flour one
Answers: 1
You know the right answer?
You have 25.0ml hcl of unknown concentration. it takes 12.5 ml of 0.2 m naoh to neutralize the acid....
Questions


Mathematics, 03.02.2021 19:20




Chemistry, 03.02.2021 19:20



Mathematics, 03.02.2021 19:20


Mathematics, 03.02.2021 19:20


Mathematics, 03.02.2021 19:20

Mathematics, 03.02.2021 19:20


History, 03.02.2021 19:20

History, 03.02.2021 19:20


Mathematics, 03.02.2021 19:20

English, 03.02.2021 19:20



