
Chemistry, 29.01.2020 22:03 EinsteinBro
Calculate the volume of a 1.420 m naoh solution required to titrate 34.55 ml of a 1.500 m h3po4 solution.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:10
Agas diffuses 1/7 times faster than hydrogen gas (h2). what is the molar mass of the gas? 100.10 g/mol 98.78 g/mol 86.68 g/mol 79.98 g/mol
Answers: 3

Chemistry, 22.06.2019 05:00
Cucl2 + 2nano3 cu(no3)2 + 2nacl what is the percent yield of nacl if 31.0 g of cucl2 reacts with excess nano3 to produce 21.2 g of nacl? 49.7% 58.4% 63.6% 78.7%
Answers: 1

Chemistry, 22.06.2019 09:30
Which ocean zone has the most abundant primary producer and why a) the abyssopelagic zone ,du to the absence of light and cold water temperatureb) the bathypelagic zone, due to the absence of light and cold water temperaturec) the mesopelagic zone ,due to uts high light availability and warm water temperature d) the epipelagic zone,due to its high light availability and warm water temperature
Answers: 3

Chemistry, 22.06.2019 09:40
In the lab, ammonia was mixed with water to form ammonium hydroxide. what is/are the reactant(s)? o water and ammonia o ammonia o ammonium hydroxide need
Answers: 2
You know the right answer?
Calculate the volume of a 1.420 m naoh solution required to titrate 34.55 ml of a 1.500 m h3po4 solu...
Questions

Advanced Placement (AP), 01.07.2019 19:00




Mathematics, 01.07.2019 19:00

Business, 01.07.2019 19:00


Health, 01.07.2019 19:00

Mathematics, 01.07.2019 19:00


Biology, 01.07.2019 19:00

Mathematics, 01.07.2019 19:00

Mathematics, 01.07.2019 19:00

Mathematics, 01.07.2019 19:00

Mathematics, 01.07.2019 19:00

Mathematics, 01.07.2019 19:00




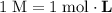 .
.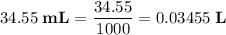 .
.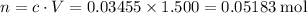 .
.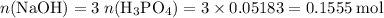 .
.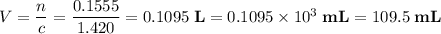 .
.

