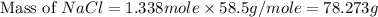Chemistry, 31.01.2020 23:04 sjjarvis53211
Part 1. a chemist reacted 15.0 liters of f2 gas with nacl in the laboratory to form cl2 and naf. use the ideal gas law equation to determine the mass of nacl that reacted with f2 at 280. k and 1.50 atm.
f2 + 2nacl → cl2 + 2naf
part 2. explain how you would determine the mass of sodium chloride that can react with the same volume of fluorine gas at stp.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:20
One or more substances changing into one or more substances is an example of a
Answers: 1

Chemistry, 22.06.2019 17:30
Energy defines the different "states" of matter. in no more than 3 sentences, describe the amount of kinetic energy that each of the 3 states of matter possesses and relate that to the atom/molecular motion of each "state".
Answers: 2


Chemistry, 23.06.2019 01:10
Can someone check my work 98 5.05 acids and bases for this assignment you will be comparing acids and bases. the chart below will you organize the information needed: acids bases chemical properties (2) deodorant detergent vinger dish soap physical properties (2) orange juice toilet cleaner drain cleaner window cleaner ph level acid ph goes from 0-4 bases ph goes from 10-14 examples around you (2) vinger coffee lemon juice dark chocolate
Answers: 3
You know the right answer?
Part 1. a chemist reacted 15.0 liters of f2 gas with nacl in the laboratory to form cl2 and naf. use...
Questions




Mathematics, 05.11.2020 06:50


Mathematics, 05.11.2020 06:50

Mathematics, 05.11.2020 06:50

Mathematics, 05.11.2020 06:50

History, 05.11.2020 06:50


Mathematics, 05.11.2020 06:50

History, 05.11.2020 06:50

Mathematics, 05.11.2020 06:50

Mathematics, 05.11.2020 06:50

Computers and Technology, 05.11.2020 06:50


Social Studies, 05.11.2020 06:50


History, 05.11.2020 06:50

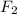 gas by using ideal gas equation.
gas by using ideal gas equation.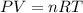
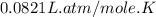
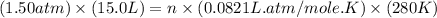
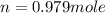
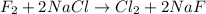
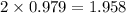 moles of NaCl
moles of NaCl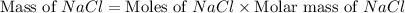
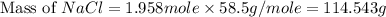
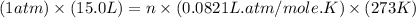
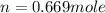
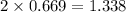 moles of NaCl
moles of NaCl