
Chemistry, 14.12.2019 04:31 araminaara691
The freezing of methane is an exothermic change. what best describes the temperature conditions that are likely to make this a spontaneous change? any temperature, because entropy increases during freezing. any temperature, because entropy decreases during freezing. low temperature only, because entropy decreases during freezing. high temperature only, because entropy increases during freezing.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:50
2points why do scientists need governmental funding? o a. government politicians ask all the important scientific questions. o b. scientists have to pay taxes to the government on the money they make. o c. the cost of doing scientific research can be very high. o d. the government is controlled by scientists. submit
Answers: 3

Chemistry, 22.06.2019 05:00
Agas can holds 2.0 gal of gasoline. what is this quantity in cubic centimeters?
Answers: 2

Chemistry, 22.06.2019 10:00
Water's surface tension and heat storage capacity are accounted for by its a) orbitals b) weight c) hydrogen bonds d) mass e) size
Answers: 2

Chemistry, 22.06.2019 10:10
When water dissociates, each water molecule splits into a hydroxide ion and a) h 3 o + b) a hydrogen atom c) a hydrogen ion d) h 2 o e) oh —
Answers: 2
You know the right answer?
The freezing of methane is an exothermic change. what best describes the temperature conditions that...
Questions



Mathematics, 28.01.2021 18:10


Chemistry, 28.01.2021 18:10

English, 28.01.2021 18:10

Mathematics, 28.01.2021 18:10


Law, 28.01.2021 18:10

Mathematics, 28.01.2021 18:10



Mathematics, 28.01.2021 18:10

Computers and Technology, 28.01.2021 18:10

Business, 28.01.2021 18:10


Chemistry, 28.01.2021 18:10

Mathematics, 28.01.2021 18:10


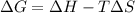
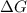 = change in Gibb's free energy
= change in Gibb's free energy 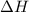 = change in enthalpy
= change in enthalpy = change in entropy
= change in entropy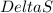 is decreasing and its sign is negative. This reaction is an exothermic reaction, which means that the
is decreasing and its sign is negative. This reaction is an exothermic reaction, which means that the ![-ve=-ve-[T(-ve)]\\\\-ve=-ve+T](/tpl/images/0418/2588/a4550.png)
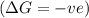 , the temperature must be low.
, the temperature must be low.

