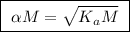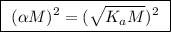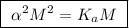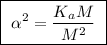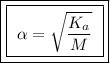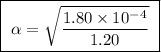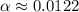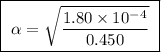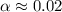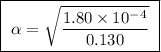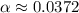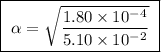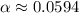Chemistry, 23.09.2019 03:00 marelinatalia2000
Calculate the percent ionization of formic acid solutions having the following concentrations. a) 1.20mb)0.450mc)0.130md)5.10 x 10^-2

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 14:30
Connect the whole numbers on the periodic table to indicate what they represent?
Answers: 3

Chemistry, 22.06.2019 20:30
Select all the correct answers.which compounds have the empirical formula ch20? (multiple answers)a.c2h4o2b.c3h603c.ch2o2d.c5h1005e.c6h1206
Answers: 2

Chemistry, 23.06.2019 02:00
Anitrogen atom and an oxygen atom combine chemically to form nitric oxide. what is nitric oxide?
Answers: 1
You know the right answer?
Calculate the percent ionization of formic acid solutions having the following concentrations. a) 1....
Questions

French, 24.02.2022 08:50



History, 24.02.2022 08:50

English, 24.02.2022 08:50


Medicine, 24.02.2022 09:00

Biology, 24.02.2022 09:00

Mathematics, 24.02.2022 09:00

History, 24.02.2022 09:00



Biology, 24.02.2022 09:00


Mathematics, 24.02.2022 09:00

Mathematics, 24.02.2022 09:00




Social Studies, 24.02.2022 09:00

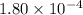
![\boxed{ \ [H^+] = \alpha M \ }](/tpl/images/0253/7357/36fb6.png) and
and ![\boxed{ \ [H^+] = \sqrt{K_a M} \ }](/tpl/images/0253/7357/a6bf9.png)