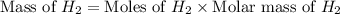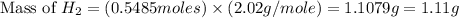Chemistry, 28.01.2020 10:31 imlexi12393
If 40.0 g of hcl react with an excess of magnesium metal, what is the theoretical yield of hydrogen?
a. 1.11 g
b. 2.22 g
c. 52.2 g
d. 104 g

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:00
The rules of engagement (roe) working group is often used to (select all that apply.)
Answers: 2

Chemistry, 22.06.2019 08:30
In a chemical reaction at equilibrium, the rate of the forward reaction the rate of the reverse reaction. if the rate of the forward reaction more products are formed.
Answers: 1

Chemistry, 22.06.2019 12:00
Ageochemist examines a piece of metal that he found in the soil. he performs tests to identify the metal from its density, electrical conductivity, and melting point. which statement best describes his investigation? a. he is determining physical properties that are sufficient to identify the metal.b. he is determining chemical properties that are sufficient to identify the metal.c. he is determining physical properties that are insufficient to identify the metal.d. he is determining chemical properties that are insufficient to identify the metal.
Answers: 3

Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 2
You know the right answer?
If 40.0 g of hcl react with an excess of magnesium metal, what is the theoretical yield of hydrogen?...
Questions

Mathematics, 08.05.2020 20:57



History, 08.05.2020 20:57

Mathematics, 08.05.2020 20:57

Mathematics, 08.05.2020 20:57

Mathematics, 08.05.2020 20:57

History, 08.05.2020 20:57

History, 08.05.2020 20:57







Mathematics, 08.05.2020 20:57




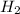 = 2.02 g/mole
= 2.02 g/mole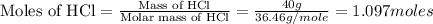
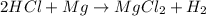
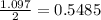 mole of hydrogen
mole of hydrogen