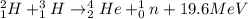The characteristics of two different types of reactions are shown below:
reaction a: an ato...

Chemistry, 21.10.2019 14:30 lindsayb2000
The characteristics of two different types of reactions are shown below:
reaction a: an atom loses electrons during the reaction.
reaction b: an atom loses protons and neutrons during the reaction.
which statement is true about the two reactions?
both reactions retain the identity of the elements.
both reactions change the identity of the elements.
reaction a produces more energy than reaction b.
reaction b produces more energy than reaction a.

Answers: 3


Another question on Chemistry


Chemistry, 21.06.2019 22:00
If 1.63 times 10 negative 4 of helium dissolves in 100.0g of water, what is the concentration in parts per million
Answers: 3

Chemistry, 22.06.2019 12:30
In france, grapes are 1.95 euros per kilogram. what is the cost of grapes, in dollars per pound, if the exchange rate is 1.14 dollars/euro? (2.6)
Answers: 3

Chemistry, 22.06.2019 14:30
According to le chatelier’s principle, a system in chemical equilibrium responds to stress by shifting the equilibrium in a direction that reduces the stress. normalizes the stress. increases the stress. changes the stress.
Answers: 1
You know the right answer?
Questions

Mathematics, 06.01.2021 01:40

Mathematics, 06.01.2021 01:40


Mathematics, 06.01.2021 01:40


Mathematics, 06.01.2021 01:40

English, 06.01.2021 01:40

Mathematics, 06.01.2021 01:40

English, 06.01.2021 01:40


English, 06.01.2021 01:40


Social Studies, 06.01.2021 01:40


Social Studies, 06.01.2021 01:40



Mathematics, 06.01.2021 01:40

Mathematics, 06.01.2021 01:40

 .
.