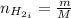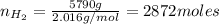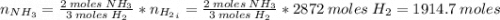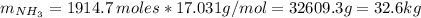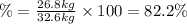Chemistry, 28.09.2019 15:10 victoriacarr638
Areaction vessel for synthesizing ammonia by reacting nitrogen and hydrogen is charged with 5.79 kg of h2 and excess n2. a total of 26.8 kg of nh3 are produced. what is the percent yield of the reaction?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:20
Which of the following statements is false regarding aromaticity? a. the compound must be cyclic b. the compound must be fully conjugated c. the compound must be planar d.the number of electrons in the pi system must satisfy the hückel 4n+2 rule e. the compound must have a neutral charge
Answers: 2

Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 3

Chemistry, 23.06.2019 00:10
Find the missing probability in the table below a.0.10 b.40 c.0.80 d. 0.20
Answers: 2

You know the right answer?
Areaction vessel for synthesizing ammonia by reacting nitrogen and hydrogen is charged with 5.79 kg...
Questions


Mathematics, 10.06.2020 10:57


History, 10.06.2020 10:57



Mathematics, 10.06.2020 10:57

Advanced Placement (AP), 10.06.2020 10:57

Computers and Technology, 10.06.2020 10:57

Arts, 10.06.2020 10:57

Health, 10.06.2020 10:57

Geography, 10.06.2020 10:57




Mathematics, 10.06.2020 10:57


History, 10.06.2020 10:57


Mathematics, 10.06.2020 10:57

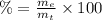 (2)
(2)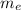 : is the experimental (or actual) mass = 26.8 kg
: is the experimental (or actual) mass = 26.8 kg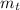 : is the theoretical mass
: is the theoretical mass