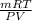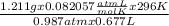Chemistry, 29.01.2020 03:59 svarner2001
Agas collected from a reaction has a mass of 1.211 g and occupies a volume of 0.677 l. the temperature in the laboratory is 296 k, and the air pressure is 0.987 atm. calculate the molar mass of the gas.
a) 23.7 g/mol
b) 44.0 g/mol
c) 58.5 g/mol
d) 82.3 g/mol

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Seltzer water is created by placing water under pressure with carbon dioxide gas. which of the following statements best describe seltzer water: a. the solution will be slightly acidic b. the solution will be slightly basic. the solution will be strongly acidic. d. the solution will be strongly basic. e. the solution will be neutral
Answers: 3

Chemistry, 22.06.2019 09:00
Look at the spectrums of a star moving towards earth and a motionless star. which of these is a correct inference that can be draw from the observation of the two spectrums? (2 points) the spectrum of a motionless star is difficult to be viewed separately using oridinary telescopes. the spectrum of a motionless star is identical to the spectrum of a star which moves towards earth. the spectrum of a star shifts towards the red region when the star moves towards earth. the spectrum of a star shifts towards the blue region when the star moves towards earth.
Answers: 2

Chemistry, 22.06.2019 12:50
The number at the end of an isotope’s name is the number.
Answers: 1

Chemistry, 22.06.2019 23:00
What is the number of neutrons in an atom with atomic mass of 35
Answers: 2
You know the right answer?
Agas collected from a reaction has a mass of 1.211 g and occupies a volume of 0.677 l. the temperatu...
Questions


Mathematics, 16.12.2020 20:00

Computers and Technology, 16.12.2020 20:00

Mathematics, 16.12.2020 20:00

Mathematics, 16.12.2020 20:00




Chemistry, 16.12.2020 20:00

Mathematics, 16.12.2020 20:00

Mathematics, 16.12.2020 20:00


History, 16.12.2020 20:00

English, 16.12.2020 20:00

Mathematics, 16.12.2020 20:00

Mathematics, 16.12.2020 20:00




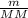 ) RT → MM =
) RT → MM =