
Chemistry, 28.09.2019 09:20 xmanavongrove55
Redox titrations are used to determine the amounts of oxidizing and reducing agents in solution. for example, a solution of hydrogen peroxide, h2o2, can be titrated against a solution of potassium permanganate, kmno4. the following equation represents the reaction: 2kmno4(aq)+h2o2(aq)+3h2so4(aq)→3o2( g)+2mnso4(aq)+k2so4(aq)+4h2o(l) a certain amount of hydrogen peroxide was dissolved in 100. ml of water and then titrated with 1.68 m kmno4. how much h2o2 was dissolved if the titration required 14.3 ml of the kmno4 solution?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:20
What is the ima of the 1 st class lever in the graphic given? 2 3 0.5
Answers: 1

Chemistry, 22.06.2019 03:40
Chemical kinetics what was the rate of reaction in trial 3? choose the closest answer.
Answers: 3

Chemistry, 22.06.2019 09:30
1. explain hydrogen peroxide, h 2 o 2 properties and decomposition reaction. 2. describe how each of the following natural cycles plays a part in earth’s climate system. (a) the water cycle (b) the carbon cycle
Answers: 1

You know the right answer?
Redox titrations are used to determine the amounts of oxidizing and reducing agents in solution. for...
Questions

English, 09.09.2020 23:01

Mathematics, 09.09.2020 23:01

Mathematics, 09.09.2020 23:01

History, 09.09.2020 23:01

Mathematics, 09.09.2020 23:01

English, 09.09.2020 23:01

Mathematics, 09.09.2020 23:01

Mathematics, 09.09.2020 23:01

Mathematics, 09.09.2020 23:01

Mathematics, 09.09.2020 23:01

Mathematics, 09.09.2020 23:01

English, 09.09.2020 23:01

Social Studies, 09.09.2020 23:01

History, 09.09.2020 23:01

Mathematics, 09.09.2020 23:01

Biology, 09.09.2020 23:01

Mathematics, 09.09.2020 23:01

Mathematics, 09.09.2020 23:01

Mathematics, 09.09.2020 23:01

Mathematics, 09.09.2020 23:01

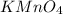 solution = 1.68 M
solution = 1.68 M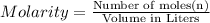
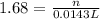
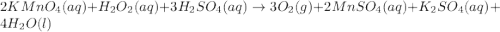
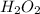 , then 0.0240 moles of KMnO_4 will react with :
, then 0.0240 moles of KMnO_4 will react with :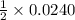 moles of
moles of 

