
Chemistry, 05.10.2019 22:20 pinklover2002
According to the kinetic-molecular theory, the average kinetic energy of gas particles depends on which factor?
volume
pressure
temperature
number of particles

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:40
The formation of a solid, also known as a is an indication of a chemical change. precipitate particulate particle powder
Answers: 3

Chemistry, 22.06.2019 22:00
Plz ill give u brainliest which of the following steps is not likely to take place during cellular respiration? a.oxygen combines with carbon of simple sugar. b. energy molecule transfers energy to cells. d. energy is used up.
Answers: 3

Chemistry, 22.06.2019 23:30
How many grams of ammonia would be produced by the decomposition of 16.93 mlof hydrazine? (the density of hydrazine is 1.021g/ml)
Answers: 3

Chemistry, 23.06.2019 02:00
The bone of a dinosaur and the imprint of a leaf are examples of which kind of fossils? a) index b) body c) amber d) trace
Answers: 1
You know the right answer?
According to the kinetic-molecular theory, the average kinetic energy of gas particles depends on wh...
Questions

World Languages, 30.08.2021 14:00

Mathematics, 30.08.2021 14:00

Health, 30.08.2021 14:00

English, 30.08.2021 14:00



English, 30.08.2021 14:00

English, 30.08.2021 14:00



English, 30.08.2021 14:00

Mathematics, 30.08.2021 14:00

Business, 30.08.2021 14:20

Mathematics, 30.08.2021 14:20

Geography, 30.08.2021 14:20

Social Studies, 30.08.2021 14:30

Health, 30.08.2021 14:30

Biology, 30.08.2021 14:30


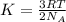
 = Avogadro's number
= Avogadro's number

