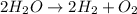Chemistry, 06.12.2019 14:31 diegobebe503
The decomposition of water into hydrogen gas h2 and oxygen gas o2 can be modeled by the balanced chemical equation
a) h2 + o2 → h2o
b) h2o → h2 + o2
c) 2h2 + o2 → 2h2o
d) 2h2o → 2h2 + o2

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:50
Acompound contains c, h, and o atoms. when 1.130 g of the compound is burned in oxygen, 1.064 g co2 and 0.3631 g h2o are produced. what is the empirical formula of this compound?
Answers: 1

Chemistry, 22.06.2019 11:50
Which of the following statements about hybrid orbitals is or are true? choose all that apply. choose all that apply. under sp2 hybridization, the large lobes point to the vertices of an equilateral triangle. after an atom undergoes sp hybridization there is one unhybridized p orbital on the atom. the angle between the large lobes of sp3 hybrids is 109.5∘
Answers: 2

Chemistry, 22.06.2019 15:20
An alloy contains 66 g of pure zinc. what is the percentage of zinc in the alloy? express your answer to two significant figures and include the appropriate units.
Answers: 1

Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 2
You know the right answer?
The decomposition of water into hydrogen gas h2 and oxygen gas o2 can be modeled by the balanced che...
Questions

Biology, 01.07.2021 14:00



English, 01.07.2021 14:00

Mathematics, 01.07.2021 14:00

History, 01.07.2021 14:00


Physics, 01.07.2021 14:00


Health, 01.07.2021 14:00

English, 01.07.2021 14:00

Mathematics, 01.07.2021 14:00


Mathematics, 01.07.2021 14:00


Mathematics, 01.07.2021 14:00

Mathematics, 01.07.2021 14:00

Mathematics, 01.07.2021 14:00


Mathematics, 01.07.2021 14:00