
Chemistry, 05.02.2020 05:54 snowflakekitty001
What would the final freezing point of water be if 3 mol of sugar were added to 1 kg of water (kf = 1.86c/(mol/kg) for water and i = 1 for sugar)?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:00
Look at the reaction below: ca(hco3)2 --> caco3 + co2 + h2o first, balance the reaction. once balanced, use dimensional analysis or another method to find out how many moles of carbon dioxide will be produced if we start with 16.5 moles of calcium bicarbonate (calcium hydrogen carbonate). = mol of co2 number needs to be reported to three significant figures.
Answers: 1

Chemistry, 21.06.2019 17:40
If 10.0 ml of the solution on the right are withdrawn from the 100 ml beaker and diluted again in a similar manner, what is the new concentration? m nacl
Answers: 2

Chemistry, 21.06.2019 22:30
Often on a topographic map, every fifth contour line is darkened. what is this line called? a. key b.slope c.benchmark d. index contour
Answers: 1

Chemistry, 21.06.2019 23:10
Which statement describes both homogeneous mixtures and heterogeneous mixtures?
Answers: 1
You know the right answer?
What would the final freezing point of water be if 3 mol of sugar were added to 1 kg of water (kf =...
Questions

Mathematics, 31.01.2021 14:00

Mathematics, 31.01.2021 14:00


Mathematics, 31.01.2021 14:00


Mathematics, 31.01.2021 14:00


Mathematics, 31.01.2021 14:00


Mathematics, 31.01.2021 14:00

Mathematics, 31.01.2021 14:00

Mathematics, 31.01.2021 14:00

History, 31.01.2021 14:00


Spanish, 31.01.2021 14:00

Mathematics, 31.01.2021 14:00

Health, 31.01.2021 14:00


History, 31.01.2021 14:00

Mathematics, 31.01.2021 14:00

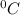
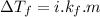
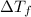 is the depression in freezing point of a solution, i is the vant hoff factor of solute,
is the depression in freezing point of a solution, i is the vant hoff factor of solute, 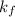 is the cryogenoscopic constant of solvent and m is molality of the solution.
is the cryogenoscopic constant of solvent and m is molality of the solution.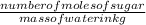 =
= 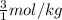 = 3 mol/kg
= 3 mol/kg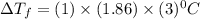 = -5.58
= -5.58

