
Chemistry, 21.01.2020 15:31 AnxiousKid
Janet observes that the shape of a dented table tennis ball is restored by heating the ball gently in warm water. which of the following gas laws is used to understand the observation made by janet and why?
the high temperature lowers volume according to boyle's law because this law describes how a gas will behave when the number of moles remains constant.
the high temperature raises volume according to boyle's law because this law describes how a gas will behave when the number of moles remains constant.
the high temperature lowers the volume of air inside the ball according to charles's law because this law describes how a gas will behave at constant pressure.
the high temperature raises the volume of air inside the ball according to charles's law because this law describes how a gas will behave at constant pressure.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:30
Calculate the change in entropy if br2(l) is converted to br2(g). s° for br2(l) = 152.2 j/(mol•k) s° for br2(g) = 245.5 j/(mol•k) s° for br(g) = 175.0 j/(mol•k)
Answers: 3

Chemistry, 22.06.2019 00:00
What is the result of multiplying (2.5 × 1010) × (2.0 × 10-7)? a. 5.0 × 103 b. 5.0 × 10-3 c. 5.0 × 1017 d. 5.0 × 10-17
Answers: 1

Chemistry, 22.06.2019 09:30
Based on its chemical properties, identify the position of each chemical family on the periodic table.
Answers: 3

Chemistry, 22.06.2019 23:30
Imagine a small synthetic vesicle made from pure phospholipids enclosing an interior lumen containing 1 mm glucose and 1 mm sodium chloride. if the vesicle is placed in pure water, which of the following happens faster? a. na+ diffuses out. b. cl– diffuses out. c. h2o diffuses in. d. glucose diffuses out. e. sodium chloride diffuses out.
Answers: 3
You know the right answer?
Janet observes that the shape of a dented table tennis ball is restored by heating the ball gently i...
Questions



Mathematics, 21.12.2020 08:20


Mathematics, 21.12.2020 08:20

History, 21.12.2020 08:20

Business, 21.12.2020 08:20

History, 21.12.2020 08:20




Mathematics, 21.12.2020 08:20

History, 21.12.2020 08:20

English, 21.12.2020 08:20


English, 21.12.2020 08:30




History, 21.12.2020 08:30

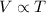 .
.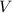 is the volume of an ideal gas, and
is the volume of an ideal gas, and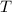 is its temperature in degrees Kelvins.
is its temperature in degrees Kelvins.

