
Chemistry, 25.09.2019 01:00 genyjoannerubiera
In part iii, the phenolphthalein indicator is used to monitor the equilibrium shifts of the ammonia/ammonium ion system. the phenolphthalein equilibrium established with water is hph(aq)(colorless) + h2o (l) h3o+ (aq) + ph-(aq)(pink or red). you compared the color of the solutions in three test tubes that initially contained 3 ml of 0.1 m ammonium hydroxide and a few drops of phenolphthalein indicator. in the first test tube, you added 1 m nh4cl dropwise. what color change was observed and what did this color change indicate about the shift in the phenolphthalein equilibrium? a. the solution turned a more intense pink or red color indicating that the phenolphthalein equilibrium shifted to the left, producing more of the pink or red colored hph.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 12:50
Assume that the variables x and y are inversely related. if k = 18, what is the value of y for each of the following points? be sure and record your data to be used in the following problem.
Answers: 2

Chemistry, 21.06.2019 15:30
219 grams of iron (iii) oxide reacts with excess carbon according to the reaction equation shown below. fe2o3 + c → fe + co2 after a scientist performs the chemical reaction they find the actual yield of iron to be 57.4 grams. calculate the percent yield of this chemical reaction.
Answers: 1

Chemistry, 21.06.2019 15:40
What is the total reduction potential of a cell in which potassium (k) is reduced and copper (cu) is oxidized? a. 2.59 v b. 3.27 v c. -3.27 v d.-2.59 v
Answers: 1

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1
You know the right answer?
In part iii, the phenolphthalein indicator is used to monitor the equilibrium shifts of the ammonia/...
Questions



Mathematics, 05.10.2019 06:00






Physics, 05.10.2019 06:00











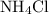 is a salt soluble in water.
is a salt soluble in water. 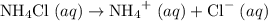 .
.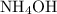 .
. 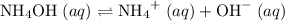 .
.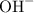 and
and 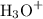 ions.
ions.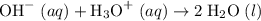 .
.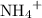 ions in the solution. Some of the
ions in the solution. Some of the 

