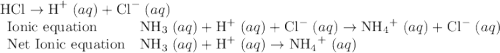Chemistry, 18.12.2019 10:31 lezapancakes13
Which of the chemical equations below are acid-base (proton transfer) reactions? select all that apply. mg(oh)2 (s) + h2so4 (aq) → mgso4 (s) + 2 h2o (l) fe(no3)3 (aq) + 3 koh (aq) → fe(oh)3 (s) + 3 kno3 (aq) zn (s) + cu(no3)2 (aq) → zn(no3)2 (aq) + cu (s) mg (s) + cu(no3)2 (aq) --> mg(no3)3 (aq) + cu (s) hcl (aq) + koh (aq) → kcl (aq) + h2o (l) nh3 (aq) + hcl (aq) → nh4cl (aq)?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Achef makes salad dressing by mixing oil, vinegar, and spices, as shown. which type of matter is the salad dressing?
Answers: 1

Chemistry, 22.06.2019 09:30
The chart shows the bid provided by four contractors to complete a job. which contractor is the most cost-effective?
Answers: 3


Chemistry, 22.06.2019 14:30
Chemistry worksheet - i am not sure what they are asking for exactly?
Answers: 1
You know the right answer?
Which of the chemical equations below are acid-base (proton transfer) reactions? select all that ap...
Questions

Mathematics, 19.06.2021 14:30

Mathematics, 19.06.2021 14:30




Health, 19.06.2021 14:30

Physics, 19.06.2021 14:30


Mathematics, 19.06.2021 14:30

English, 19.06.2021 14:30

Physics, 19.06.2021 14:30

Advanced Placement (AP), 19.06.2021 14:30


Mathematics, 19.06.2021 14:30

Mathematics, 19.06.2021 14:50

Biology, 19.06.2021 14:50


Mathematics, 19.06.2021 14:50

Biology, 19.06.2021 14:50

Chemistry, 19.06.2021 14:50