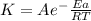Chemistry, 30.09.2019 22:00 GingerSnaps
The rate constant (k) for a reaction was measured as a function of temperature. a plot of lnk versus 1/t(in k) is linear and has a slope of −9.20×103 k . you may want to reference (pages 642 - 648) section 14.5 while completing this problem. part a calculate the activation energy for the reaction.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:50
If the mass of the products measured 120g what would the mass of the reactants a. 30g b. 60g c. 120g d. 240g
Answers: 1


Chemistry, 22.06.2019 04:00
Which of the following ocean acidification? are the most likely side effects of a ph less than 7.0 in the ocean b. more metal salts altering the ocean chemistry c. dissolution of the shells of marine organisms d. both a & b e. all of the above.
Answers: 3

Chemistry, 22.06.2019 20:30
Select all the correct answers.which compounds have the empirical formula ch20? (multiple answers)a.c2h4o2b.c3h603c.ch2o2d.c5h1005e.c6h1206
Answers: 2
You know the right answer?
The rate constant (k) for a reaction was measured as a function of temperature. a plot of lnk versus...
Questions



Spanish, 10.12.2020 03:00


History, 10.12.2020 03:00

Social Studies, 10.12.2020 03:00

History, 10.12.2020 03:00


Computers and Technology, 10.12.2020 03:00

Mathematics, 10.12.2020 03:00

Advanced Placement (AP), 10.12.2020 03:00


Mathematics, 10.12.2020 03:00

History, 10.12.2020 03:00

Social Studies, 10.12.2020 03:00

Biology, 10.12.2020 03:00

Social Studies, 10.12.2020 03:00

Mathematics, 10.12.2020 03:00

History, 10.12.2020 03:00