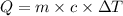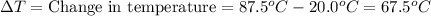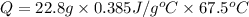Chemistry, 21.10.2019 18:00 miriammayo12345
Copper metal has a specific heat of 0.385 j/g. c. calculate the amount of heat required to raise the temperature of 22.8g of copper metal from 20.0c to 87.5c

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 18:30
Which sample at stp has the same number of atoms as 18 liters of ne at stp
Answers: 1


Chemistry, 22.06.2019 21:40
A5 mole sample of liquid acetone is converted to a gas at 75.0°c. if 628 j are required to raise the temperature of the liquid to the boiling point, 15.600 kj are required to evaporate the liquid, and 712 j are required to raise the final temperature to 75.0°c, what is the total energy required for the conversion?
Answers: 3

Chemistry, 23.06.2019 01:20
How can parts of a solution be separated by chromatography?
Answers: 1
You know the right answer?
Copper metal has a specific heat of 0.385 j/g. c. calculate the amount of heat required to raise the...
Questions

World Languages, 02.08.2019 13:20

Biology, 02.08.2019 13:20

Spanish, 02.08.2019 13:20

Mathematics, 02.08.2019 13:20

Mathematics, 02.08.2019 13:20

Mathematics, 02.08.2019 13:20

Mathematics, 02.08.2019 13:20



Mathematics, 02.08.2019 13:20

Social Studies, 02.08.2019 13:20

History, 02.08.2019 13:20

Mathematics, 02.08.2019 13:20

Mathematics, 02.08.2019 13:20



Mathematics, 02.08.2019 13:20


Social Studies, 02.08.2019 13:20