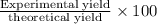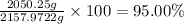Chemistry, 29.01.2020 23:48 jossfajardo50
Honors stoichiometry activity worksheet
instructions: in this laboratory activity, you will taste test two samples of just lemons lemonade for taste quality. then you will analyze lemonade production data for percent yield and excess ingredients. complete each section of this worksheet, and submit it to your instructor for grading.
activity two: just lemons, inc. production
here's a one-batch sample of just lemons lemonade production. determine the percent yield and amount of leftover ingredients for lemonade production and place your answers in the data chart. hint: complete stoichiometry calculations for each ingredient to determine the theoretical yield. complete a limiting reactant-to-excess reactant calculation for both excess ingredients.
water 946.36 g sugar 196.86 g lemon juice 193.37 g lemonade 2050.25
percent yield ? leftover ingredients ?
just lemons lemonade recipe equation:
2 water + sugar + lemon juice = 4 lemonade
mole conversion factors:
1 mole of water = 1 cup = 236.59 g
1 mole of sugar = 1 cup = 225 g
1 mole of lemon juice = 1 cup = 257.83 g
1 mole of lemonade = 1 cup = 719.42 g

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:30
7. using your knowledge of colligative properties explain whether sodium chloride or calcium chloride would be a more effective substance to melt the ice on a slick sidewalk. use 3 – 4 sentences in your explanation. 8. when a 2.5 mol of sugar (c12h22o11) are added to a certain amount of water the boiling point is raised by 1 celsius degree. if 2.5 mol of aluminum nitrate is added to the same amount of water, by how much will the boiling point be changed? show all calculations leading to your answer or use 3 – 4 sentences to explain your answer. 9. if 5.40 kcal of heat is added to 1.00 kg of water at 100⁰c, how much steam at 100⁰c is produced? show all calculations leading to an answer. 10. the freezing of water at 0⁰c can be represented as follows: h2o (l) ↔ h2o(s) the density of liquid water is 1.00 g/cm3. the density of ice is 0.92 g/cm3. in 3 – 4 sentences explain why applying pressure causes ice to melt.
Answers: 1

Chemistry, 22.06.2019 11:00
Freezing and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 22.06.2019 13:10
Select the correct answer a modure consists of glucose and water. what is the percent composition of glucose in the mixture if it contains 1.3 moles of glucose (cho total mass of the mature is 276 grams? ) and the a 1775
Answers: 1

Chemistry, 22.06.2019 13:50
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3
You know the right answer?
Honors stoichiometry activity worksheet
instructions: in this laboratory activity, you w...
instructions: in this laboratory activity, you w...
Questions

Mathematics, 23.07.2021 09:00




Mathematics, 23.07.2021 09:00


Computers and Technology, 23.07.2021 09:00



Computers and Technology, 23.07.2021 09:00

English, 23.07.2021 09:00


English, 23.07.2021 09:00

Health, 23.07.2021 09:00

Mathematics, 23.07.2021 09:00


Mathematics, 23.07.2021 09:00



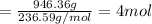
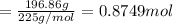
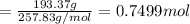
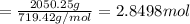
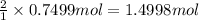 of water
of water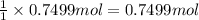 of water
of water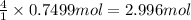 of lemonade
of lemonade