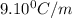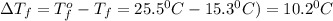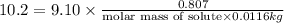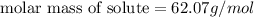Chemistry, 04.02.2020 20:46 Envious1552
Tert-butyl alcohol is a solvent with a kf of 9.10 ∘c/m and a freezing point of 25.5 ∘c. when 0.807 g of an unknown colorless liquid was dissolved in 11.6 g of tert-butyl alcohol, the solution froze at 15.3 ∘c. which of the following is most likely the identity of this unknown liquid? tert-butyl alcohol is a solvent with a of 9.10 and a freezing point of 25.5 . when 0.807 of an unknown colorless liquid was dissolved in 11.6 of tert-butyl alcohol, the solution froze at 15.3 .which of the following is most likely the identity of this unknown liquid? ethylene glycol (molar mass = 62.07 g/mol)1-octanol (molar mass = 130.22 g/mol)glycerol (molar mass = 92.09 g/mol)2-pentanone (molar mass = 86.13 g/mol)1-butanol (molar mass = 74.12 g/mol)

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 13:00
Which of the following are good traits of a hypothesis? it will be able to be testedit can predict an outcomeit will explain the observationsall of these
Answers: 2

Chemistry, 22.06.2019 17:00
Complete each row of the table below by filling in the missing prefix or missing exponent.
Answers: 1

Chemistry, 22.06.2019 19:00
Structure of the atoms: discovery of the nucleus in 1909i need answering all of these questions
Answers: 3
You know the right answer?
Tert-butyl alcohol is a solvent with a kf of 9.10 ∘c/m and a freezing point of 25.5 ∘c. when 0.807 g...
Questions

Mathematics, 15.02.2020 07:24



Chemistry, 15.02.2020 07:25

History, 15.02.2020 07:25


History, 15.02.2020 07:25

Mathematics, 15.02.2020 07:25




Mathematics, 15.02.2020 07:26

Mathematics, 15.02.2020 07:26

English, 15.02.2020 07:26



Mathematics, 15.02.2020 07:27

Chemistry, 15.02.2020 07:27

Computers and Technology, 15.02.2020 07:27

Chemistry, 15.02.2020 07:27

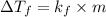
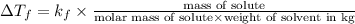
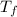 = change in freezing point
= change in freezing point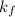 = freezing point constant=
= freezing point constant=