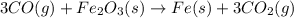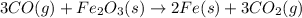Chemistry, 28.09.2019 15:10 prinxcess1206
Which is the next logical step in balancing the given equation? 3co(g) + fe2o3(s) fe(s) + 3co2(g)
a. place the coefficient 2 in front of iron(iii) oxide.
b. leave it alone, as it is already balanced.
c. place the coefficient 2 in front of elemental iron.
d. replace the coefficient 3 in carbon monoxide with 6.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 16:30
An atom with 7 protons, 6 neutrons, and 7 electrons has an atomic mass of amu. (enter a whole number.) numerical answers expected! answer for blank 1:
Answers: 3

Chemistry, 22.06.2019 21:50
If e is the symbol for an element, which two of the following symbols represent isotopes of the same element? 1. e2. e3. ea.1 and 2c.1 and 4b.3 and 4d.2 and 3
Answers: 2

Chemistry, 22.06.2019 22:30
Which one of the following bonds would you expect to be the most polar? a) b–h b) n–h c) p–h d) al–h e) c–h
Answers: 1

Chemistry, 23.06.2019 08:40
The activation energy for this reaction is 75 kj·mol–1. the enzyme catalase (found in blood) lowers the activation energy to 8.0 kj·mol–1. at what temperature would the non-catalyzed reaction need to be run to have a rate equal to that of the enzyme-catalyzed reaction at 25°c?
Answers: 2
You know the right answer?
Which is the next logical step in balancing the given equation? 3co(g) + fe2o3(s) fe(s) + 3co2(g) <...
Questions



Mathematics, 17.04.2020 03:53


Mathematics, 17.04.2020 03:53

History, 17.04.2020 03:53


Chemistry, 17.04.2020 03:54

History, 17.04.2020 03:54


History, 17.04.2020 03:54





Mathematics, 17.04.2020 03:54

Biology, 17.04.2020 03:54

Mathematics, 17.04.2020 03:54