
Chemistry, 29.01.2020 17:45 firdausmohammed80
Reviewing for a test - need an answer and explanation.
base your answer to the following question on the information below.
the reaction between aluminum and an aqueous solution of gold (i) sulfate is represented by the unbalanced equation below.
al (s) + au2so4 (aq) -> al2(so4)3 (aq) + au (s)
determine the total mass of au sproduced when 6.52 grams of al reacts completely with 3.58 grams of au2so4 to produce 5.95 grams of al2(so4)3.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 23:30
With the largest atoms and the smallest number of valence electrons and with the smallest atoms and the greatest number of valence electrons are the most reactive. a. nonmetals; metals b. nonmetals; transition elements c. transition elements; metals d. metals; nonmetals
Answers: 3

Chemistry, 22.06.2019 23:30
The density of benzene at 15 °c is 0.8787 g/ml. calculate the mass of 0.1500 l of benzene at this temperature. enter your answer in terms of grams
Answers: 2

Chemistry, 23.06.2019 10:30
Most ionic compouds are crystalline solids at room temperature. true falseionic compounds are electrically neutral. true falseionic compounds generally have low melting points. true falsewhen melted, ionic compounds do not conduct electricity. true falsethe electrostatic attraction between an anion and a cation is an ionic bond. true false
Answers: 1
You know the right answer?
Reviewing for a test - need an answer and explanation.
base your answer to the following...
base your answer to the following...
Questions

Mathematics, 27.01.2020 18:31



Mathematics, 27.01.2020 18:31




History, 27.01.2020 18:31

History, 27.01.2020 18:31

Social Studies, 27.01.2020 18:31





History, 27.01.2020 18:31

Geography, 27.01.2020 18:31

Geography, 27.01.2020 18:31

English, 27.01.2020 18:31

English, 27.01.2020 18:31

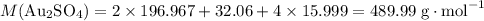 .
.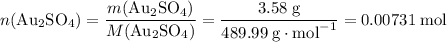 .
.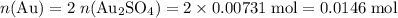 .
.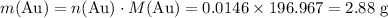 .
.

