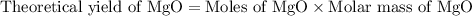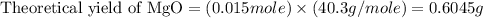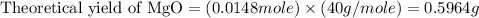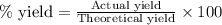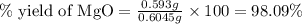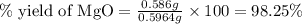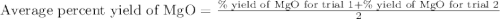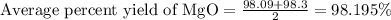Chemistry, 10.11.2019 17:31 shortty1111
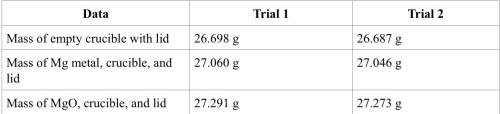
1. calculate the actual yield of magnesium oxide for each trial.
trial 1:
trial 2:
2. magnesium is the limiting reactant in this experiment. calculate the theoretical yield of mgo for each trial.
trial 1:
trial 2:
3. determine the percent yield of mgo for your experiment for each trial.
trial 1:
trial 2:
4. determine the average percent yield of mgo for the two trials.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 13:00
In a copper wire, a temperature increase is the result of which of the following
Answers: 1

Chemistry, 22.06.2019 13:30
How many moles is 14.5 cm^3 of platinum? the density of platinum is 21.45 g/cm^3.
Answers: 1

Chemistry, 22.06.2019 16:50
Which element is least likely to undergo a chemical reaction
Answers: 3

Chemistry, 23.06.2019 01:30
If a particle has z = 25 and 23 electrons, what is its charge?
Answers: 2
You know the right answer?
1. calculate the actual yield of magnesium oxide for each trial.
trial 1:
trial...
trial 1:
trial...
Questions



Mathematics, 25.03.2020 05:50





Physics, 25.03.2020 05:50

Mathematics, 25.03.2020 05:50


Health, 25.03.2020 05:50


Mathematics, 25.03.2020 05:50


Mathematics, 25.03.2020 05:50


Advanced Placement (AP), 25.03.2020 05:50

Mathematics, 25.03.2020 05:50


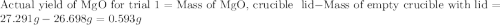
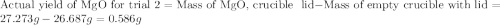
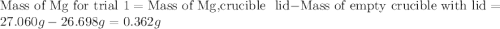
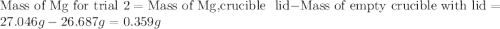
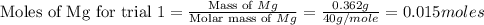
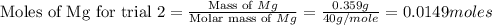
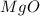 for trial 1 and trial 2.
for trial 1 and trial 2.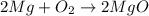
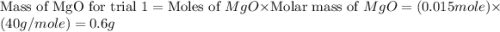
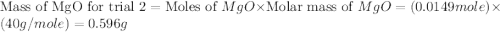
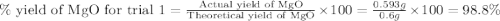
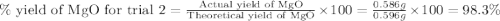

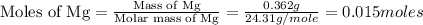
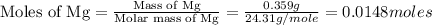
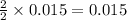 moles of MgO.
moles of MgO.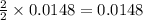 moles of MgO.
moles of MgO.