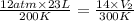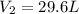Chemistry, 17.10.2019 15:20 naiquawhite
If i initially have a gas at a pressure of 12 atm, volume of 23 liters, and temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Asolution of sodium hydroxide was titrated against a solution of sulfuric acid. how many moles of sodium hydroxide would react with 1 mole of sulfuric acid?
Answers: 2

Chemistry, 22.06.2019 04:00
Seltzer water is created by placing water under pressure with carbon dioxide gas. which of the following statements best describe seltzer water: a. the solution will be slightly acidic b. the solution will be slightly basic. the solution will be strongly acidic. d. the solution will be strongly basic. e. the solution will be neutral
Answers: 3

Chemistry, 22.06.2019 08:50
If two atoms are bonded to a central atom with no lone pairs,how will they be arranged
Answers: 3

You know the right answer?
If i initially have a gas at a pressure of 12 atm, volume of 23 liters, and temperature of 200 k, an...
Questions




History, 28.06.2019 12:30

Physics, 28.06.2019 12:30


History, 28.06.2019 12:30








History, 28.06.2019 12:30

English, 28.06.2019 12:30


Mathematics, 28.06.2019 12:30


Health, 28.06.2019 12:30

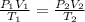
 = initial pressure of gas = 12 atm
= initial pressure of gas = 12 atm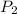 = final pressure of gas = 14 atm
= final pressure of gas = 14 atm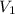 = initial volume of gas = 23 L
= initial volume of gas = 23 L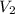 = final volume of gas = ?
= final volume of gas = ?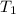 = initial temperature of gas = 200K
= initial temperature of gas = 200K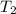 = final temperature of gas = 300K
= final temperature of gas = 300K