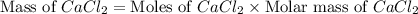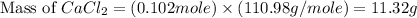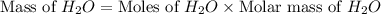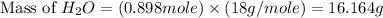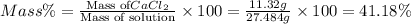Chemistry, 09.01.2020 00:31 nehakarakkattu
"an aqueous cacl2 solution has a vapor pressure of 83.1mmhg at 50 ∘c. the vapor pressure of pure water at this temperature is 92.6 mmhg. what is the concentration of cacl2 in mass percent? "

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:20
Which formula can be used to calculate the molar mass of ammonia (nh3)? molar mass of n + molar mass of h 3 × molar mass of n + molar mass of h molar mass of n + 3 × molar mass of h 3 × molar mass of n + 3 × molar mass of h
Answers: 1

Chemistry, 22.06.2019 14:30
Which of the following is not one of the steps in the scientific method a. hypothesize b. summarize c. analyze d. familiarize
Answers: 3

Chemistry, 22.06.2019 22:00
The volume of an unknown substance in a sealed glass jar is 50 milliliters. the volume of the jar is 200 milliliters. which state of matter could the substance be?
Answers: 2

Chemistry, 23.06.2019 19:00
How long does it take light from the nearest star other than the sun to reach earth? a) less than 1 second b) about 1 hour c) about 1 month d) about 4 years
Answers: 1
You know the right answer?
"an aqueous cacl2 solution has a vapor pressure of 83.1mmhg at 50 ∘c. the vapor pressure of pure wat...
Questions


History, 25.02.2020 21:27
















Computers and Technology, 25.02.2020 21:27


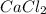 in mass percent is, 41.18 %
in mass percent is, 41.18 %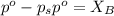
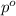 = vapor pressure of the pure component (water) = 92.6 mmHg
= vapor pressure of the pure component (water) = 92.6 mmHg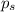 = vapor pressure of the solution = 83.1 mmHg
= vapor pressure of the solution = 83.1 mmHg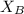 = mole fraction of solute,
= mole fraction of solute, 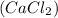
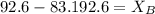
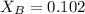
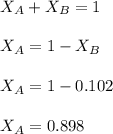
 .
.