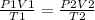Chemistry, 21.06.2019 17:00 Luzperez09
Initially, the balloon had 3.0 liters of gas at a pressure of 400 kpa and was at a temperature of 294 k. if the balloon is cooled to 277 k and its volume decreased to 1 l, what will the new pressure in the balloon be?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
One does not belong why? ice, gold ,wood ,diamond and table salt
Answers: 1

Chemistry, 22.06.2019 11:40
Consider this equilibrium: n29) + o2(g) + 2no(c).nitrogen gas and oxygen gas react when placed in a closed container. as the reaction proceeds towards equilibrium, what happens to the rate of thereverse reaction?
Answers: 1

Chemistry, 22.06.2019 13:30
What are the chemical names of these compounds? ke: mg3n2: reset next
Answers: 1

Chemistry, 22.06.2019 14:30
Calculate the mass of carbon in 97.0 g of sucrose c12h22o11
Answers: 3
You know the right answer?
Initially, the balloon had 3.0 liters of gas at a pressure of 400 kpa and was at a temperature of 29...
Questions

English, 02.09.2019 10:50


Business, 02.09.2019 10:50


Mathematics, 02.09.2019 10:50


Chemistry, 02.09.2019 10:50

Social Studies, 02.09.2019 10:50

Business, 02.09.2019 10:50

History, 02.09.2019 10:50




English, 02.09.2019 10:50

Physics, 02.09.2019 10:50



Business, 02.09.2019 10:50

Mathematics, 02.09.2019 10:50