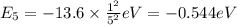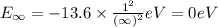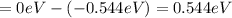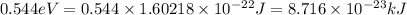Chemistry, 22.06.2019 14:00 luisaareli6298
Calculate the energy required to ionize a hydrogen atom to an excited state where the electron is initially in the n = 5 energy level. report your answer in kilojoules

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:30
Find the empirical formula of each of the following compounds. given mass or for each element in a sample of the compound 3,611 g ca; 6.389 g c1
Answers: 1

Chemistry, 22.06.2019 05:00
When you mate two plants together the terms is called? answer it fast as possible plz! i have a test tomorrow!
Answers: 1

Chemistry, 22.06.2019 22:30
Molecular iodine, i2(g), dissociates into iodine atoms at 625 k with a first-order rate constant of 0.271 s−1. part a part complete what is the half-life for this reaction?
Answers: 3

Chemistry, 22.06.2019 22:50
At the current rate, a graph of carbon dioxide produced by fossil fuels over time would slope upward slope downward be horizontal be vertical
Answers: 3
You know the right answer?
Calculate the energy required to ionize a hydrogen atom to an excited state where the electron is in...
Questions




Business, 17.02.2021 22:40

Social Studies, 17.02.2021 22:40




Mathematics, 17.02.2021 22:40

Mathematics, 17.02.2021 22:40





Mathematics, 17.02.2021 22:40


Biology, 17.02.2021 22:40


Biology, 17.02.2021 22:40

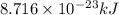 .
.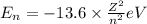
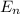 = energy of
= energy of 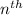 orbit
orbit