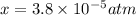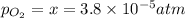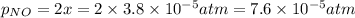Chemistry, 22.06.2019 20:00 denaemarie02
Nitrogen dioxide decomposes according to the reaction 2 no2(g) ⇌ 2 no(g) + o2(g) where kp = 4.48 × 10−13 at a certain temperature. if 0.70 atm of no2 is added to a container and allowed to come to equilibrium, what are the equilibrium partial pressures of no(g) and o2(g)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Which element forms an ionic bond with flourine? 1) fluorine 2) carbon 3) potassium 4) oxygen
Answers: 1

Chemistry, 22.06.2019 07:00
Indicate whether the specified alkyl halides will form primarily substitution products, only elimination products, both substitution and elimination products, or no products when they react with sodium methoxide. 1-bromobutane 1-bromo-2-methylpropane 2-bromobutane 2-bromo-2-methylpropane
Answers: 2

Chemistry, 22.06.2019 10:40
If an area has high air pressure and low humidity, what type of weather will it most likely have? plz !
Answers: 1

You know the right answer?
Nitrogen dioxide decomposes according to the reaction 2 no2(g) ⇌ 2 no(g) + o2(g) where kp = 4.48 × 1...
Questions


Mathematics, 26.01.2021 01:00

Health, 26.01.2021 01:00

Mathematics, 26.01.2021 01:00



Social Studies, 26.01.2021 01:00

Mathematics, 26.01.2021 01:00

History, 26.01.2021 01:00


History, 26.01.2021 01:00


Chemistry, 26.01.2021 01:00


History, 26.01.2021 01:00

Physics, 26.01.2021 01:00

Mathematics, 26.01.2021 01:00


Mathematics, 26.01.2021 01:00

Mathematics, 26.01.2021 01:00

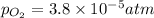
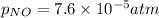
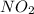 = p = 0.70 atm
= p = 0.70 atm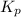 for the reaction
for the reaction 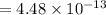
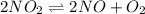
![K_p=\frac{[2x]^2[x]}{[p-2x]^2}](/tpl/images/0005/2998/5610b.png)
![4.48\times 10^{-13}=\frac{4x^3}{[0.70-2x]^2}](/tpl/images/0005/2998/9a087.png)