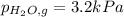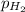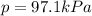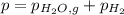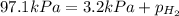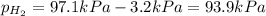When a chemist collects hydrogen gas over water, she ends up with a mixture of hydrogen and water vapor in her collecting bottle. if the pressure in the collecting bottle is 97.1 kilopascals and the vapor pressure of the water is 3.2 kilopascals, what is the partial pressure of the hydrogen?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:40
Which of these expressions are correct variations of the combined gas law? p1v1t2 = p2v2t1 both
Answers: 2

Chemistry, 22.06.2019 13:30
Ants live on acacia trees in south america. the ants feed on sugars secreted by the trees. the trees provide room for the ants to live. the ants sting any other insect or animal that comes to eat the trees. what type of relationship is this?
Answers: 1

Chemistry, 23.06.2019 01:30
Witch two conditions can limit the usefulness of the kinetic molecular theory in describing gas behavior?
Answers: 2

Chemistry, 23.06.2019 03:30
Mr. rose asked his student to draw a quadrilateral with four unequal sides. an example of this kind of quadrilateral
Answers: 1
You know the right answer?
When a chemist collects hydrogen gas over water, she ends up with a mixture of hydrogen and water va...
Questions

Mathematics, 13.09.2020 21:01

Mathematics, 13.09.2020 21:01

Mathematics, 13.09.2020 21:01

Mathematics, 13.09.2020 21:01

Mathematics, 13.09.2020 21:01

Mathematics, 13.09.2020 21:01

Mathematics, 13.09.2020 21:01

Mathematics, 13.09.2020 21:01

English, 13.09.2020 21:01

Mathematics, 13.09.2020 21:01

Mathematics, 13.09.2020 21:01

Mathematics, 13.09.2020 21:01

Mathematics, 13.09.2020 21:01

Chemistry, 13.09.2020 21:01

Mathematics, 13.09.2020 21:01

Mathematics, 13.09.2020 21:01

Mathematics, 13.09.2020 21:01

Mathematics, 13.09.2020 21:01

Mathematics, 13.09.2020 21:01

Mathematics, 13.09.2020 21:01