
Chemistry, 23.06.2019 13:30 michelle8978
Why hydrochloric acid neutralized first when you titrate a mixture of hcl& ch3cooh against standard sodium hydroxide

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 23:30
Why do oxygen have a strong attractive force for electrons
Answers: 2

Chemistry, 23.06.2019 00:00
Predict the relative bond lengths of the three carbon-oxygen bonds in the carbonate ion (co2−3). what would you expect the charge to be on each oxygen? match the words in the left column to the appropriate blanks in the sentences on the right. make certain each sentence is complete before submitting your answer.
Answers: 3


Chemistry, 23.06.2019 06:00
Complete the sentences to best explain the ranking.match the words below to the appropriate blanks in the sentences.a less polar bondhigher molar massion-dipole forcesstronger intermolecular forcesdipole-dipole forcesdispersion forceshydrogen bonding1. h2s and h2se exhibit the following intermolecular forces:.2. therefore, when comparing h2s and h2se the one with a has a higher boiling point .3. the strongest intermolecular force exhibited by h2o is . therefore, when comparing h2se and h2o the one with has a higher boiling point.
Answers: 1
You know the right answer?
Why hydrochloric acid neutralized first when you titrate a mixture of hcl& ch3cooh against stand...
Questions


Mathematics, 02.01.2022 14:00


Mathematics, 02.01.2022 14:00





Mathematics, 02.01.2022 14:00


Mathematics, 02.01.2022 14:00


Mathematics, 02.01.2022 14:00



Mathematics, 02.01.2022 14:00


Mathematics, 02.01.2022 14:00


Mathematics, 02.01.2022 14:00

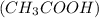 . The given mixture is a mixture of strong acid and a weak acid.
. The given mixture is a mixture of strong acid and a weak acid.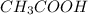 is titrated against NaOH (strong base), strong acid will get neutralized first because it is completely dissociated in the solution and can be easily neutralized.
is titrated against NaOH (strong base), strong acid will get neutralized first because it is completely dissociated in the solution and can be easily neutralized.

