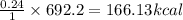Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 2

Chemistry, 22.06.2019 09:10
Which class of molecules functions as chemical signals? hormones water carbohydrates proteins
Answers: 1


Chemistry, 23.06.2019 09:30
Where are the noble gases located in the periodic table? a. in the center b. on the left side c. in the upper right corner d. on the far right side
Answers: 1
You know the right answer?
The combustion of 45.0 g of methane (natural gas) releases 2498 kj of heat energy. what amount of en...
Questions


History, 24.03.2020 03:22

Mathematics, 24.03.2020 03:22




Mathematics, 24.03.2020 03:22











History, 24.03.2020 03:22

History, 24.03.2020 03:22

Law, 24.03.2020 03:22

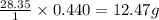
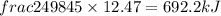 of heat energy
of heat energy