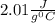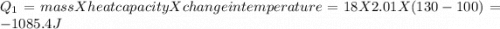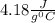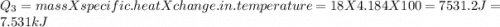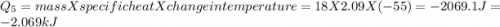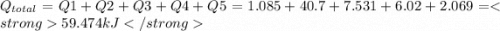Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
Which sentence best describes the formation of igneous rock? a- lava on the surface dries up and makes arock b_melted rocks cools and forms crystals c_rocks under tremendous heat and pressure d_magma is melted rock underground
Answers: 1


Chemistry, 22.06.2019 05:00
1)each group 16 element has how many valence electrons? ( )4 ( )6 ( )8 ( )16 2)how many dots appear in the dot structure for calcium ion, ca2+? ( )zero ( )one ( )two ( )eight 3) which of the following atoms forms a cation to obtain an octet of outer shell electrons? ( )magnesium ( )oxygen ( )fluorine ( )helium 4) an al3+ ion contains 13 protons and 10 electrons. ( )true ( )false 5) valence and non-valence electrons are represented in lewis dot structures. ( )true ( )false
Answers: 3

You know the right answer?
How much heat (in kj) is evolved in converting 1.00 mol of steam at 130.0 ∘c to ice at -55.0 ∘c? th...
Questions


Mathematics, 22.01.2021 02:00

Mathematics, 22.01.2021 02:00

Mathematics, 22.01.2021 02:00



Geography, 22.01.2021 02:00

Mathematics, 22.01.2021 02:00

English, 22.01.2021 02:00

Computers and Technology, 22.01.2021 02:00

Mathematics, 22.01.2021 02:00

Mathematics, 22.01.2021 02:00

Advanced Placement (AP), 22.01.2021 02:00

Mathematics, 22.01.2021 02:00




Social Studies, 22.01.2021 02:00

English, 22.01.2021 02:00

Mathematics, 22.01.2021 02:00

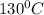 to
to 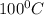 = Q1
= Q1![0^{0}C[/tex=Q3]4) conversion of water to ice=Q45) cooling of ice from [tex]0^{0}C](/tpl/images/0009/5828/c5d88.png) to
to 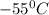 =Q5
=Q5