
Chemistry, 23.06.2019 22:30 McKenzie8409
Consider the combustion of ethylene: c2h4(g)+3o2(g)→2co2(g)+2h2o(g) if the concentration of c2h4 is decreasing at the rate of 3.6×10−2 m/s , what is the rate of change in the concentration of co2? express the rate in molarity per second to two significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:00
Ill give brainiestif one neutron initiates a fission event that produces two neutrons in the products, how many new reactions can now be initiated? if each of the neutrons produced in the first fission event then initiates a fission event that produces one neutron in the products, how many new reactions can now be initiated by each neutron? how many neutrons in total were produced by the two fission events described?
Answers: 2

Chemistry, 22.06.2019 12:10
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 13:00
Asubstance is a good conductor of electricity which of the following best explains a probable position of the substance in a periodic table
Answers: 3

Chemistry, 22.06.2019 19:20
The equation picture below shows which type of nuclear reaction u 235 + n x e 134 + sr 100 + 2n
Answers: 1
You know the right answer?
Consider the combustion of ethylene: c2h4(g)+3o2(g)→2co2(g)+2h2o(g) if the concentration of c2h4 is...
Questions

Mathematics, 01.01.2020 23:31

Geography, 01.01.2020 23:31

Mathematics, 01.01.2020 23:31

Mathematics, 01.01.2020 23:31

Mathematics, 01.01.2020 23:31

Mathematics, 01.01.2020 23:31

Biology, 01.01.2020 23:31



Mathematics, 01.01.2020 23:31

Chemistry, 01.01.2020 23:31


Chemistry, 01.01.2020 23:31


Mathematics, 01.01.2020 23:31



Mathematics, 01.01.2020 23:31


![-d\frac{[C_2H_4]}{dt}=-\frac{1}{3}d\frac{O_2}{dt}=\frac{1}{2}d\frac{CO_2}{dt}=\frac{1}{2}d\frac{H_2O}{dt}](/tpl/images/0009/5909/8a85c.png)
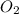 is 2, so it is written as
is 2, so it is written as 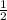 .
. ![-d\frac{[C_2H_4]}{dt}=\frac{1}{2}d\frac{CO_2}{dt}](/tpl/images/0009/5909/590ad.png)
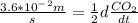
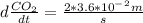
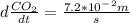
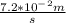 .
. 

