
Chemistry, 30.11.2019 08:31 SuperWoman9172
Carbon disulfide burns in oxygen to yield car- bon dioxide and sulfur dioxide according to the chemical equation cs2(l) 3 o2(g) −→ co2(g) 2 so2(g). if 0.91 mol of cs2 is combined with 1.52 mol of o2, identify the limiting reactant.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 13:00
One of the hopes for solving the world's energy problem is to make use of the fusion reaction 21h +31h --> 42he + 10n + energy how much energy is released when 1 mol of deuterium is fused with 1 mol of tritium according to the above reaction? the masses of the atoms and the neutrons are as follows: 21h = 2.0140 amu 31h = 3.01605 amu 42he = 4.002603 amu 10n = 1.008665 amu. the speed of light is 2.9979 x 108 m/s.
Answers: 1

Chemistry, 22.06.2019 19:30
How might this scientific phenomena be explained? a paper clip floats on water.
Answers: 1

Chemistry, 22.06.2019 21:40
A5 mole sample of liquid acetone is converted to a gas at 75.0°c. if 628 j are required to raise the temperature of the liquid to the boiling point, 15.600 kj are required to evaporate the liquid, and 712 j are required to raise the final temperature to 75.0°c, what is the total energy required for the conversion?
Answers: 3

Chemistry, 23.06.2019 06:00
Give one example of a pure (exact) number and of an estimated (measured) number.
Answers: 2
You know the right answer?
Carbon disulfide burns in oxygen to yield car- bon dioxide and sulfur dioxide according to the chemi...
Questions


English, 27.10.2021 14:00

Mathematics, 27.10.2021 14:00



Mathematics, 27.10.2021 14:00


Geography, 27.10.2021 14:00

History, 27.10.2021 14:00


Mathematics, 27.10.2021 14:00



History, 27.10.2021 14:00

SAT, 27.10.2021 14:00



Mathematics, 27.10.2021 14:00

Mathematics, 27.10.2021 14:00

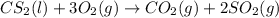
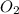 reacts with 1 mole of
reacts with 1 mole of 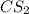
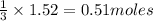 of
of 

