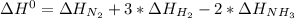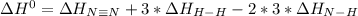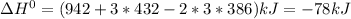Chemistry, 24.06.2019 21:30 gwoodbyrne
Given: n2 + 3h2 → 2nh3 bond bond energy (kj/mol) n≡n 942 h–h 432 n–h 386 use the bond energies to calculate the change in enthalpy for the reaction. the enthalpy change for the reaction is kilojoules.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:40
Three alkali metals in group 1 are a. calcium, strontium, barium b. boron, aluminum, gallium c. sodium, potassium, rubidium d. fluorine, iodine, chlorine
Answers: 1

Chemistry, 22.06.2019 14:30
Aroom with dimensions 7.00m×8.00m×2.50m is to be filled with pure oxygen at 22.0∘c and 1.00 atm. the molar mass of oxygen is 32.0 g/mol. how many moles noxygen of oxygen are required to fill the room? what is the mass moxygen of this oxygen?
Answers: 1

Chemistry, 22.06.2019 18:10
Measurements that have similar values are: a. usually accurate b. sometimes accurate c. always accurate d. never accurate
Answers: 1

Chemistry, 23.06.2019 01:00
Atoms contain subatomic particles called protons and neutrons. when these protons and neutrons spilt, a lot of energy is released
Answers: 3
You know the right answer?
Given: n2 + 3h2 → 2nh3 bond bond energy (kj/mol) n≡n 942 h–h 432 n–h 386 use the bond energies to c...
Questions

Mathematics, 23.02.2021 20:10

Biology, 23.02.2021 20:10


Arts, 23.02.2021 20:10


Biology, 23.02.2021 20:10

Mathematics, 23.02.2021 20:10




History, 23.02.2021 20:10



Mathematics, 23.02.2021 20:10

Mathematics, 23.02.2021 20:10




Mathematics, 23.02.2021 20:10

Health, 23.02.2021 20:10

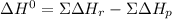 (2)
(2)