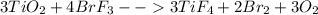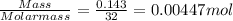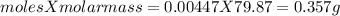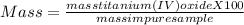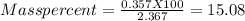Chemistry, 25.06.2019 02:00 Gearyjames8
One method for determine the purity of a sample of titanium ( iv) oxide, an important industrial chemical, is to combine the sample with bromine trifluoride to produce titanium ( iv) fluoride, liquid bromine , and oxygen has. suppose 2.367g of an impure sample( impure meaning that the sample has titanium (iv) oxide as well as other “ stuff” in it ) evolves 0.143 g of oxygen gas. what is the mass percent of titanium ( iv) oxide in the impure sample?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
When curium-242 is bombarded with an alpha particle, two products are formed, one of which is a nudge on. what is the other product
Answers: 3

Chemistry, 22.06.2019 02:00
What is the maximum number of electrons that an atomic orbital can contain?
Answers: 1

Chemistry, 22.06.2019 16:00
No copying 15 pts how does a free-body diagram tell you about the net force on an object?
Answers: 2

Chemistry, 22.06.2019 18:10
The atom fluorine generally will become stable through the formation of an ionic chemical compound by accepting electron(s) from another atom. this process will fill its outer energy level of electrons.
Answers: 1
You know the right answer?
One method for determine the purity of a sample of titanium ( iv) oxide, an important industrial che...
Questions







Chemistry, 27.06.2019 11:40









Geography, 27.06.2019 11:40

Mathematics, 27.06.2019 11:40


English, 27.06.2019 11:40

Social Studies, 27.06.2019 11:40