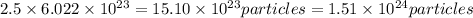Chemistry, 25.06.2019 05:30 svandewalle
How many o2 particles are in 2.50 of o2 at standard temperature and pressure (stp)? a. 4.15 x 10^22 particles b. 2.41 x 10^23 particles c. 5.02 x 10^23 particles d. 1.51 x 10^24 particles can you explain the answer ?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 05:00
Agas can holds 2.0 gal of gasoline. what is this quantity in cubic centimeters?
Answers: 2

Chemistry, 22.06.2019 14:00
The content of manganese (mn) in steel was determined spectrophotometrically and with the use of the standard addition method. an unknown sample of mn from a digested steel sample gave an absorbance of 0.185 when analyzed spectrophotometrically. when 5.00 ml of solution containing 95.5 ppm mn was added to 50.0 ml of the unknown steel solution (digested sample), the absorbance was 0.248. calculate the concentration, in parts-per-million (ppm), of mn in the digested steel sample solution.
Answers: 3

Chemistry, 22.06.2019 15:30
Each of the following reactions is allowed to come to equilibrium and then the volume is changed as indicated. predict the effect (shift right, shift left, or no effect) of the indicated volume change. drag the appropriate items to their respective bins.co(g) + h2o(g) < => co2(g) + h2(g) (volume is decreased) pcl3(g) + cl2(g) < => pcl5(g) (volume is increased) caco3(s)< => cao(s) + co2(g) (volume is increased)
Answers: 1
You know the right answer?
How many o2 particles are in 2.50 of o2 at standard temperature and pressure (stp)? a. 4.15 x 10^22...
Questions


English, 18.05.2021 16:30



Mathematics, 18.05.2021 16:30





Mathematics, 18.05.2021 16:30

Mathematics, 18.05.2021 16:30

Mathematics, 18.05.2021 16:30



Mathematics, 18.05.2021 16:30

Health, 18.05.2021 16:30

Mathematics, 18.05.2021 16:30

English, 18.05.2021 16:30


Mathematics, 18.05.2021 16:30

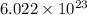 number of atoms.
number of atoms.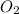 gas:
gas: