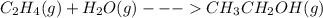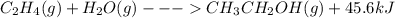When c2h4(g) reacts with h2o(g) to form ch3ch2oh(g) , 45.6 kj of energy are evolved for each mole of c2h4(g) that reacts. write a balanced thermochemical equation for the reaction with an energy term in kj as part of the equation. note that the answer box for the energy term is case sensitive. use the smallest integer coefficients possible and put the energy term (including the units) in the last box on the appropriate side of the equation. if a box is not needed, leave it blank. + + +?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:50
Significant figures are digits read directly from the measuring instrument plus one more digit, which is __ by the observer.
Answers: 2

Chemistry, 22.06.2019 10:40
Which buffer would be better able to hold a steady ph on the addition of strong acid, buffer 1 or buffer 2? explain. buffer 1: a solution containing 0.10 m nh4cl and 1 m nh3. buffer 2: a solution containing 1 m nh4cl and 0.10 m nh3
Answers: 1

Chemistry, 22.06.2019 15:20
Which description best characterizes the motion of particles in a solid?
Answers: 2

Chemistry, 22.06.2019 16:30
Asample of freon gas has a volume of 2.23 liters, a pressure of 4.85 kpa, and a temperature of -1.36°c. calculate the volume at a pressure of 1.38 kpa and a temperature of 5.5°c. (show work)
Answers: 1
You know the right answer?
When c2h4(g) reacts with h2o(g) to form ch3ch2oh(g) , 45.6 kj of energy are evolved for each mole of...
Questions





Spanish, 28.01.2020 10:31


Mathematics, 28.01.2020 10:31


Computers and Technology, 28.01.2020 10:31

History, 28.01.2020 10:31

Mathematics, 28.01.2020 10:31


Mathematics, 28.01.2020 10:31



Social Studies, 28.01.2020 10:31


Mathematics, 28.01.2020 10:31