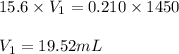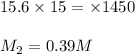Chemistry, 25.06.2019 14:00 ayowazzzgood
You have a stock solution of 15.6 m nh3. how many milliliters of this solution should you dilute to make 1450 ml of 0.210 m nh3? if you take a 15.0-ml portion of the stock solution and dilute it to a total volume of 0.600 l , what will be the concentration of the final solution?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:50
Complete the following statements to describe solids, liquids, and gases. select the correct answer from each drop-down menu. a solid a definite volume and a definite shape. a liquid a definite volume and a definite shape. a gas a definite volume and a definite shape
Answers: 1

Chemistry, 22.06.2019 19:30
Draw the lewis structure for the trisulfur s3 molecule. be sure to include all resonance structures that satisfy the octet rule.
Answers: 3

Chemistry, 22.06.2019 23:10
Using the periodic table, complete the following. element: hydrogen symbol: h₂ molecular weight: g mass of one mole: g/mol
Answers: 3

Chemistry, 23.06.2019 06:30
What type of chemical reaction occurs between silver nitrate (agno3) and copper (cu)? the equation i was given is 2agno3 + cu —> 2ag+ cu(no3)2.
Answers: 1
You know the right answer?
You have a stock solution of 15.6 m nh3. how many milliliters of this solution should you dilute to...
Questions

Social Studies, 29.07.2021 08:50






Mathematics, 29.07.2021 08:50


Mathematics, 29.07.2021 08:50

English, 29.07.2021 08:50

Mathematics, 29.07.2021 08:50

Mathematics, 29.07.2021 08:50


Mathematics, 29.07.2021 08:50

Computers and Technology, 29.07.2021 08:50

Mathematics, 29.07.2021 08:50


Mathematics, 29.07.2021 08:50

English, 29.07.2021 08:50

English, 29.07.2021 08:50

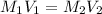
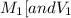 are the molarity and volume of one solution
are the molarity and volume of one solution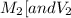 are the molarity and volume of another solution
are the molarity and volume of another solution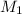 = Molarity of the stock solution = 15.6M
= Molarity of the stock solution = 15.6M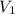 = Volume of the stock solution = ? mL
= Volume of the stock solution = ? mL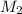 = Molarity of the diluted solution = 0.210M
= Molarity of the diluted solution = 0.210M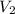 = Volume of the diluted solution = 1450 mL
= Volume of the diluted solution = 1450 mL