Chemistry, 25.06.2019 17:30 yeayeawhatever3389
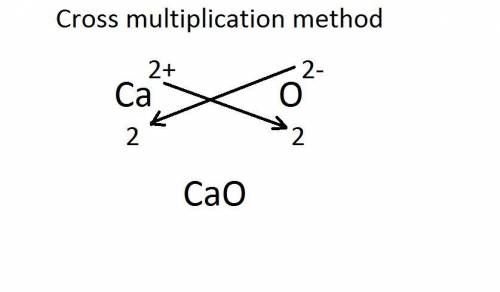
Calcium has a 2+ charge, and oxygen has a 2− charge. a lewis dot diagram should contain one calcium atom and one oxygen atom to show how these atoms form an ionic bond. true false

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Match the name of the following compound: mgso4 · h2omagnesium sulfate monohydratemagnesium (ii) sulfate monohydratemagnesium (ii) sulfate hydratemagnesium sulfate hydrate
Answers: 1

Chemistry, 22.06.2019 09:20
Which of these statements explains the difference between nuclear binding energy and the strong nuclear force ?
Answers: 3

Chemistry, 22.06.2019 22:40
Covalent bonds generally form when the bonded elements have a difference in electronegativity less than 1.5. subtract the electronegativities for the following pairs of elements and predict whether they form a covalent bond. electronegativity difference of c and c: ionic covalent electronegativity difference of mg and cl: ionic covalent
Answers: 1

Chemistry, 22.06.2019 23:00
Which of your 24 wells had indications that a chemical reaction occurred? how were you able to tell that a chemical reaction occurred? which of your 24 wells had indications that a physical reaction occurred? how were you able to tell that a physical reaction occurred? report on both mixing and evaporation. make a general statement about whether your hypotheses were validated or rejected. must your hypotheses be correct for this to be a successful laboratory?
Answers: 3
You know the right answer?
Calcium has a 2+ charge, and oxygen has a 2− charge. a lewis dot diagram should contain one calcium...
Questions

Health, 24.09.2019 10:00

Mathematics, 24.09.2019 10:00

Computers and Technology, 24.09.2019 10:00

Biology, 24.09.2019 10:10

Mathematics, 24.09.2019 10:10

Mathematics, 24.09.2019 10:10







Geography, 24.09.2019 10:10

Mathematics, 24.09.2019 10:10

Biology, 24.09.2019 10:10

Biology, 24.09.2019 10:10

Social Studies, 24.09.2019 10:10

Biology, 24.09.2019 10:10

Mathematics, 24.09.2019 10:10

Business, 24.09.2019 10:10

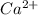 .
.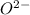 .
.