Chemistry, 25.06.2019 19:00 blakestuhan
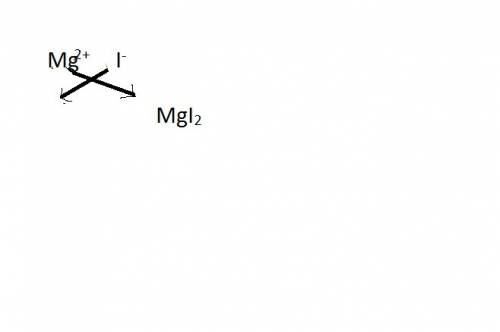
Select the properly written and balanced equation for the following chemical reaction: sodium metal and an aqueous solution of magnesium iodide react to produce an aqueous solution of sodium iodide and magnesium metal. na(s) + mgi (aq) -> nai (aq) + mg(s) na(s) + mgi2 (aq) -> nai2 (aq) + mg (s) 2na (s) + mgi2 (aq) -> 2nai (aq) + mg (s) 2na (s) + 4mgi (aq) -> 2nai2 (aq) + 4mg(s)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:10
Nitric oxide (no) can be formed from nitrogen, hydrogen and oxygen in two steps. in the first step, nitrogen and hydrogen react to form ammonia: n2(g) + 2 h_2(g) rightarrow 2 nh_3 (g) delta h = -92. kj in the second step, ammonia and oxygen react to form nitric oxide and water: 4 nh_3(g) + 5 o_2(g) rightarrow 4no(g) + 6 h_2o(g) delta h = -905. kj calculate the net change in enthalpy for the formation of one mole of nitric oxide from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 1

Chemistry, 22.06.2019 06:30
How many moles of carbon dioxide will form if 2.5 moles of c3h8 is burned
Answers: 1

Chemistry, 22.06.2019 06:40
Which statement is usually true about the relationship between activation energy and reaction rates? low activation energy barriers result in low rates. high activation energy barriers result in low rates. low activation energy barriers result in no reaction. high activation energy barriers result in no reaction.
Answers: 3

Chemistry, 22.06.2019 08:30
How does the principle of electromagnetism explain the interaction between earth’s magnetic field and the solar wind?
Answers: 1
You know the right answer?
Select the properly written and balanced equation for the following chemical reaction: sodium metal...
Questions


Biology, 04.03.2021 22:00

Mathematics, 04.03.2021 22:00

Social Studies, 04.03.2021 22:00




Mathematics, 04.03.2021 22:00

Mathematics, 04.03.2021 22:00


Mathematics, 04.03.2021 22:00

Social Studies, 04.03.2021 22:00

English, 04.03.2021 22:00

Mathematics, 04.03.2021 22:00

Social Studies, 04.03.2021 22:00

Mathematics, 04.03.2021 22:00

Mathematics, 04.03.2021 22:00

Mathematics, 04.03.2021 22:00


Mathematics, 04.03.2021 22:00


 .
.