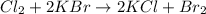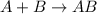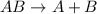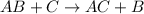Chemistry, 25.06.2019 21:00 Sprout7430
Identify the single displacement reaction. c4h12 + 7o2 ⟶ 6h2o + 4co2 2h2 + o2 ⟶ 2h2o al2s3 ⟶ 2al + 3s cl2 + 2kbr ⟶ 2kcl + br2

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:30
100 points answer quick the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1

Chemistry, 22.06.2019 04:00
Tin has ten stable isotopes. the heaviest, 124sn, makes up 5.80% of naturally occuring tin atoms. how many atoms of 124sn are present in 82.0 g of naturally occurring tin? what is the total mass of the 124sn atoms in this sample?
Answers: 3

Chemistry, 22.06.2019 15:10
Which statement describes the phase change that occurs when dry ice is placed in an open container at room temperature?
Answers: 1

You know the right answer?
Identify the single displacement reaction. c4h12 + 7o2 ⟶ 6h2o + 4co2 2h2 + o2 ⟶ 2h2o al2s3 ⟶ 2al + 3...
Questions

History, 26.06.2019 18:10

Mathematics, 26.06.2019 18:10


Geography, 26.06.2019 18:10


Geography, 26.06.2019 18:10

Geography, 26.06.2019 18:10

Physics, 26.06.2019 18:10

Physics, 26.06.2019 18:10

English, 26.06.2019 18:10





Mathematics, 26.06.2019 18:10


Biology, 26.06.2019 18:10

Health, 26.06.2019 18:10