
Chemistry, 25.06.2019 22:30 tatibean26
Consider the chemical equations shown here.2no2(g) > 2no(g) + o2(g)2no(g) > n2(g) + o2(g)n2(g) + 2o(g) > n2o4(g)what is the equation for the overall reaction obtained by adding these equations? 2no2(g) > n2o4(g)2n204(g) + 2no(g) > 2no2(g) + 02(g)n2 + o2(g) + 2no(g) > n2o4 (g)the answer is a

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
How many atoms of mg are present in 97.22 grams of mg? 6.022 × 1023 2.408 × 1024 4.818 × 1024 5.855 × 1025
Answers: 3

Chemistry, 22.06.2019 14:30
Need ! asap will mark 10 pts using the room temperature line (orange line) and your periodic table, make lists that identify the state of matter (gas, liquid, or solid) in which each element you plotted exists at room temperature. explain your answers
Answers: 1

Chemistry, 22.06.2019 21:30
What is the effect of returning nuclear reactor cooling water back into bodies of water?
Answers: 3

Chemistry, 22.06.2019 23:00
If two identical atoms are bonded,what kind of molecule is formed
Answers: 1
You know the right answer?
Consider the chemical equations shown here.2no2(g) > 2no(g) + o2(g)2no(g) > n2(g) + o2(g)n2(...
Questions

History, 05.07.2021 05:10

Mathematics, 05.07.2021 05:10

Mathematics, 05.07.2021 05:10


English, 05.07.2021 05:10

Mathematics, 05.07.2021 05:10



English, 05.07.2021 05:10


Physics, 05.07.2021 05:10



Mathematics, 05.07.2021 05:20




Mathematics, 05.07.2021 05:20



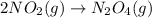
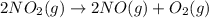 ...(1)
...(1)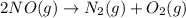 ...(2)
...(2)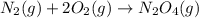 ...(3)
...(3)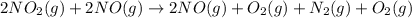
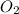 is on same side it will get added up.
is on same side it will get added up.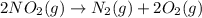 ..(4)
..(4)


