
Chemistry, 26.06.2019 04:00 gcarter1203
Match the following associations. 1. + δhexothermic endothermic 2. e2 < e¹exothermic endothermic 3. energy is absorbed exothermic endothermic 4. e2 > e¹exothermic endothermic .5- δhexothermic endothermic

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:00
6what is the importance of water on earth? a) it keeps the top layer of the geosphere cool b) it allows life to exist c) it provides ice at the poles d) it creates earth's blue color from space
Answers: 2


Chemistry, 22.06.2019 20:10
The lattice enthalpy (formation of ionic solid from ions in the gas phase) for agcl(s) is -916 kj/mol and the hydration enthalpy (dissolution of gaseous ions into water) is -850 kj/mol. how much heat (in joules) is involved in forming 1l of saturated agcl solution (1.8 × 10-4 g / 100 ml water) by dissolving agcl(s)? assume solution volume does not change much upon dissolution. the equations are given below. ag+(g) + cl−(g) æ agcl(s)
Answers: 3

Chemistry, 22.06.2019 23:30
Aweight lifter raises a 1600 n barbell to a height of 2.0 meters. how much work was done? w = fd a) 30 joules b) 3000 joules c) 320 joules d) 3200 joules
Answers: 2
You know the right answer?
Match the following associations. 1. + δhexothermic endothermic 2. e2 < e¹exothermic endothermic...
Questions

Chemistry, 10.10.2020 14:01

Mathematics, 10.10.2020 14:01



Mathematics, 10.10.2020 14:01


Computers and Technology, 10.10.2020 14:01






Mathematics, 10.10.2020 14:01

Biology, 10.10.2020 14:01

Mathematics, 10.10.2020 14:01




Mathematics, 10.10.2020 14:01

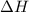 is positive then it means heat is absorbed by reactants and reaction is endothermic.
is positive then it means heat is absorbed by reactants and reaction is endothermic.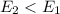 , this means energy of products is less than the energy of reactants. Therefore, it is exothermic in nature.
, this means energy of products is less than the energy of reactants. Therefore, it is exothermic in nature.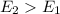 , this means energy of products is greater than the energy of reactants. Therefore, it is endothermic in nature.
, this means energy of products is greater than the energy of reactants. Therefore, it is endothermic in nature.


