
Chemistry, 26.06.2019 08:00 potternatalie90
What precipitate forms when silver nitrate and potassium chromate solutions are mixed? explain. a. ag2cro4( s) b. k2cro4( s) c. agno3( s) d. kno3( s) an answer would be really appreciated! need it as soon as possible!

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:30
What is the mass of each element in a 324.8 sample of co2
Answers: 1

Chemistry, 22.06.2019 06:40
Three alkali metals in group 1 are a. calcium, strontium, barium b. boron, aluminum, gallium c. sodium, potassium, rubidium d. fluorine, iodine, chlorine
Answers: 1

Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 3

You know the right answer?
What precipitate forms when silver nitrate and potassium chromate solutions are mixed? explain. a....
Questions


Mathematics, 05.03.2020 16:31

English, 05.03.2020 16:31


Mathematics, 05.03.2020 16:31





Mathematics, 05.03.2020 16:32



World Languages, 05.03.2020 16:32




Mathematics, 05.03.2020 16:33



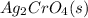
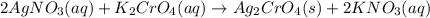
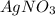 combines with potassium chromate
combines with potassium chromate 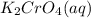 ,they undergo double displacement reaction in which exchange of ions take place.
,they undergo double displacement reaction in which exchange of ions take place.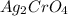 which is insoluble in water and thus formed as precipitate and potassium nitrate
which is insoluble in water and thus formed as precipitate and potassium nitrate 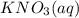 is soluble in water.
is soluble in water.

